Human Subjects Research
Institutional Review Board (IRB)
- participants’ rights and welfare are adequately protected,
- research is guided by the ethical principles of respect for persons, beneficence, and justice as set forth in the Belmont Report
- research is conducted with the highest level of expertise and integrity, and
- research complies with all applicable laws, policies and regulations.
-
As of January 24, 2022, all faculty, staff, and student researchers must complete CITI Research, Ethics, and Compliance Training prior to submitting an IRB proposal.
- Instructions for registering for CITI training
- Students are required to complete a set of 4 modules, while faculty and staff are required to complete a set of 8 modules.
- Researchers' certificates of completion must be turned in with IRB proposals.
Fall 2024 Updates
Proposals requiring full committee review will be reviewed on a rolling basis during the Fall '24 semester. Please allow at least two weeks for review of proposals requiring full committee review.
All proposals for research with human participants must go through the Institutional Review Board for Research with Human Subjects (IRB) before any data is collected. As of January 24, 2022, students, faculty, and staff working on research with human subjects must complete the CITI Research Ethics and Compliance Training.
The purpose of the IRB is to ensure that the rights of participants in human research are protected, in line with the federal guidelines (45 CFR 46). All investigators conducting research with human subjects should submit a proposal to the IRB for review, and should only conduct the research AFTER the proposal is reviewed and approved. The IRB will not grant approval for proposals submitted after the data has already been collected.
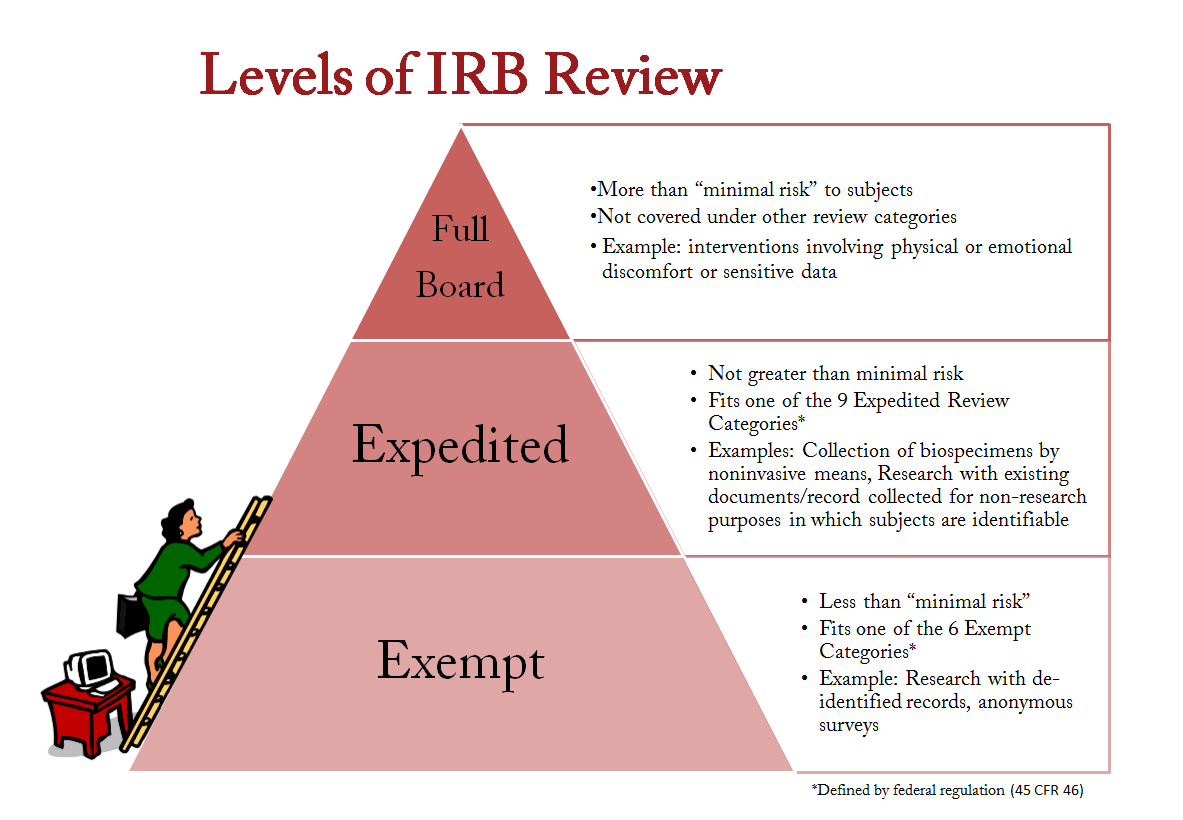
Please fill out the IRB application (Form A) for new proposals. Instructions for selecting the review category can be found on the last two pages of the document. These instructions do not need to be submitted with the IRB proposal. You can also review an example IRB.
Please send electronic versions of your IRB proposals to IRBFREEwashcoll. Make sure to have all forms (the IRB proposal, consent form, debriefing form, measures, etc.) within one document if possible. Make sure to attach CITI certificates for all researchers. We are no longer accepting hard copies of submissions.
Exempt/expedited proposals will typically be reviewed within five (5) business days of the date they are submitted to the review board.
Proposals requiring full committee review will be reviewed during biweekly meetings (every two weeks). The dates of these meetings are listed above.
Proposals submitted over breaks will take additional time.
Following review, the primary investigator will receive email notification of the outcome of the review. The proposal may be approved, or it may require changes or additional information that must then be resubmitted for review. For all of these reasons, investigators are strongly encouraged to submit their exempt/expedited proposals at least three (3) weeks before they intend to start the project, and full committee proposals at least four (4) weeks before the data collection start date.
Most research proposal are approved for 1 year, unless otherwise specified. For research that extends beyond 1 year, the investigator should submit a proposal for renewal (see next section).
Note: In accordance with the federal guidelines for research with human subjects, you must obtain informed consent from all of your research subjects. If the subject is a minor (age 17 or under), you must obtain written consent from a parent or guardian.
An important duty of the Institutional Review Board is to maintain a record of all active research protocols.
Renewal after 1 year: Projects that have been previously approved by the Board must be re-submitted for renewal either 1 year after initial approval, or if the research project is modified. Please complete and submit Section I of Form C for project renewals.
Longitudinal Research: In instances of longitudinal research, the investigator must submit a brief paragraph that summarizes the findings of the study and the justifications for renewal. Please complete and submit Section II of Form C for longitudinal project renewals.
Washington College students who are 17 and younger, but would like to participate in research, can have their parent/guardian fill out the Parental/Guardian Consent Form. This form must be returned to the chair of the IRB (Dr. Tia Murphy, tmurphy2FREEwashcoll) before participating in research.
If you have participated in research and feel that you were treated in an unprofessional manner or have concerns about your rights as a research participant, you can contact the Principal Investigator or the chair of the Review Board for Research with Human Subjects (Dr. Tia Murphy, tmurphy2FREEwashcoll).
- Form A - IRB application for new proposals
- Form B - IRB application for studies involving drug use, shock, threat, deception, embarassment, or other invasive procedures
- Form C - IRB application for project renewal and/or revision
- Consent Guidelines
- Parent/Guardian Consent Form
- Sample IRB Proposal
- Descriptions of Review Categories
- Creating an Electronic Signature
Professor Tia Murphy, Chair of IRB
Email: tmurphy2FREEwashcoll
Professor Brian Scott, Social Science Representative
Email: bscott3FREEwashcoll
Professor Aileen Tsui, Humanities and Fine Arts Representative
Email: atsui2FREEwashcoll
Professor Jen Wanat, Natural Sciences Representative
Email: jwanat2FREEwashcoll